Ministry of Defence
|
|
Market Exploration: High Consequence Infectious Disease Medical Evacuation
The Defence and Security Accelerator is scoping capabilities, technologies and initiatives for High Consequence Infectious Disease Medical Evacuation.
Summary
On behalf of the United Kingdom (UK) Ministry of Defence (MOD) and Royal Air Force (RAF), the Defence and Security Accelerator (DASA) is running a Market Exploration to understand the current market of capabilities and technologies related to High Consequence Infectious Disease (HCID) Medical Evacuation (MEDEVAC) in order to identify, develop and deliver an enhanced capability for Defence. The Market Exploration will provide knowledge of what potential solutions already exist, novel solutions in development and areas that may require further investment by MOD.
Whilst potentially successful technologies and concepts may be exploited within a future acquisition programme, please note that this request for information is not a commitment to subsequently launch a formal DASA competition.
Background
The Deployable Air Isolator Team, is a joint Department of Health and Social Care and MOD asset and can transport patients with possible or confirmed HCID. The current Air Transportable Isolator (shown above and detailed within CDC Paper published in Jan 19), provides the capability for strategic [1] Aeromedical Evacuation (AE) of a patient with a HCID (please note that at the time of launch COVID-19 is not considered an HCID, so this is not a COVID-19-specific requirement)[2] .
HCIDs are divided into contact and airborne groups; contact HCIDs spread by contact with an infected patient or by indirect contact with contaminated materials. Airborne HCIDs are spread by respiratory droplets or aerosol transmission, in addition to contact routes of transmission. The Public Health England list of HCID is at this link: UK Gov HCID guidance.
Medical Capability (Air) are leading the review of future capability options, which can in part or as a whole, transfer patients from a point of infection/ exposure (or confirmation of infection) anywhere in the world to a UK-based dedicated Specialist Medical Treatment Facility (MTF). We are seeking additional capability to facilitate the AE of patients with airborne and/or contact HCID. The new capability, or systems within it, should facilitate both strategic and tactical [3] AE, thus enabling the movement of HCID patients throughout the Operational Patient Care Pathway (OPCP), as shown below:
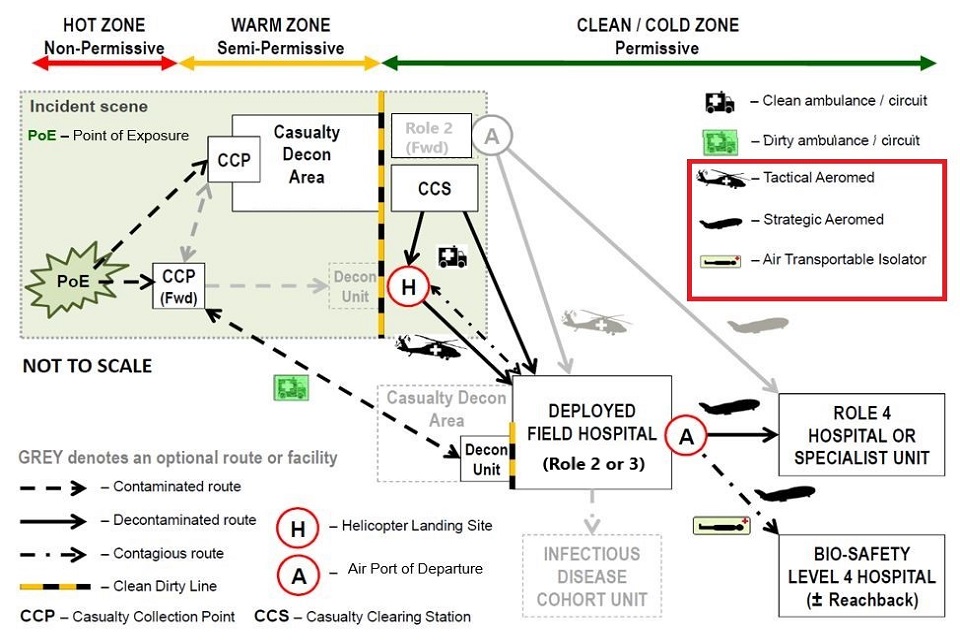
Figure 1- Operational Patient Care Pathway. The technology should be suitable for use on the Tactical Aeromed, Strategic Aeromed and Air Transportable Isolator pathways on the diagram. Extract from Joint Chemical Biological Radiological and Nuclear (CBRN) Briefing.
Enhancements to the current capability must take into account the constraints and environmental challenges that air (fixed wing and rotary) and vehicular platforms bring in terms of; options for ruggedization [4] , for maintaining patient comfort, prevention of aircraft/support personnel contamination, communications, access for treatment during transportation, extended transit times and space limitations. The technology should be suitable for use on the Tactical Aeromed, Strategic Aeromed and Air Transportable Isolator pathways on the diagram.
Essential requirements
- system can transfer patients with a confirmed, or suspected, HCID (airborne and/or contact HCID)
- system can as a whole or in part be used strategically and tactically
- the system allows for access for treatment
- the system will prevent, so far as is reasonably possible, contamination of aircraft and support personnel
What we want
We are particularly interested in capabilities, technologies, initiatives and novel approaches which, in part or as a whole, can transfer HCID patients through the OPCP from a point of infection (or confirmation of) to a UK Role 4 [5] hospital or dedicated specialist centre. This will enable strategic MEDEVAC (from an operational theatre) and tactical MEDEVAC (within a theatre of operations and at the most advanced units or elements of the OPCP).
Evidence of trials of operational use for capabilities or technologies will be welcomed in the Market Exploration. Novel initiatives and approaches, new technologies and extant capabilities will be considered.
What we don’t want
We are not interested in literature reviews, paper-based studies, consultancy, non-technical solutions or marginal improvements to existing capabilities.
This is not a competition; therefore, we are not asking for costed proposals at this stage. This is a market engagement request as an information exercise; we do not commit to subsequently launching a formal DASA competition.
How to submit a Market Exploration Submission to DASA
Responses to this Market Exploration must be submitted via the DASA submission service, for which you will need to register. We recommend you use a Google Chrome browser to access the DASA submission service.
You will be asked for a title and short summary of your innovation, along with questions related to your organisation, your idea and technology maturity. We are seeking to understand what and how much further development is required for a complete solution to meet requirements, or whether a combination of separate solutions is required. The information you provide will assist in developing a statement of requirements for potential future activities.
Submissions must be submitted by midday on Thursday 24th June 2021. Unfortunately we are unable to accept any submissions after this point.
Please only provide details of one product/capability per submission. If you have a number of potential solutions, then please submit multiple forms.
If you have any questions, then please email accelerator@dstl.gov.uk with “HCID AE Market Exploration” in the subject line.
How we use your information
Information you provide to us in a Market Exploration Submission, that is not already available to us from other sources, will be handled in-confidence. By submitting a Market Exploration Submission, you are giving us permission to keep and use the information for our internal purposes and to provide the information onwards, in-confidence, within UK Government. The Defence and Security Accelerator will not use or disclose the information for any other purpose, without first requesting permission to do so.
Footnotes
- Strategic AE is the movement of patients from a theatre of operations to a Role 4 medical treatment facility (MTF) in the UK or a Partner Nation Role 4 equivalent. Defined as the phase of MEDEVAC that provides air transport for patients from a MTF within an area of operations to a MTF outside the areas of operations.
- As of 19 March 2020 public health bodies in the UK determined that COVID-19 is no longer considered to be a high consequence infectious disease (HCID) in the UK.
- Tactical MEDEVAC is the movement of patients between deployed medical treatment facilities, within the theatre of operations.
- To withstand aircraft vibrations.
- A Role 4 MTF offers the full spectrum of definitive medical care that cannot be deployed to a Theatre of Operations or will be too time consuming to be conducted in Theatre. Role 4 MTFs normally provide definitive care, specialist surgical and medical procedures, reconstructive surgery and rehabilitation.
Related content
Collection
Brexit
Original article link: https://www.gov.uk/government/news/market-exploration-high-consequence-infectious-disease-medical-evacuation


