Spinraza access agreement extended
4 Jul 2019 11:34 AM
More children with the rare genetic disorder spinal muscular atrophy (SMA) can now be treated with Spinraza after NICE yesterday (3 July) published amended draft guidance following a proposal for extending the terms of the managed access agreement (MAA) between NHS England and Biogen for funding it.
The company made the proposal taking into consideration further clinical evidencei. The MAA has also been refined in light of a number of requests for clarification.
The most significant change to the MAA is that it will now include paediatric patients who have recently (in the previous 12 months) lost the ability to walk independently. This replaces the previous criteria that patients, who had previously gained ambulation, should still be able to walk independently at the start of their treatment with Spinraza.
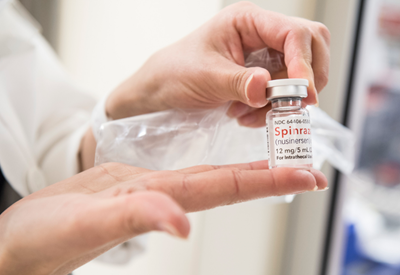
Spinraza (also called nusinersen) is the first treatment that targets the underlying cause of SMA.
SMA affects the nerves in the spinal cord controlling movement. This causes muscle weakness, progressive loss of movement, and difficulty breathing and swallowing.
People with the most severe forms of SMA usually die before the age of 2. Without nusinersen the condition is managed through supportive care which aims to minimise the impact of disability, address complications and improve quality of life.
It is estimated there are there are between 600 and 1200 children and adults in the UK living with SMA.
Yesterday’s announcement follows the agreement of a revised deal between NHS England and Biogen which will see the NHS fund treatment with Spinraza for a time-limited period, allowing further data to be collected on its effectiveness.
Meindert Boysen, director of the Centre for Health Technology Evaluation at NICE, yesterday said:
“Having considered new evidence about the effectiveness of Spinraza in people who have lost the ability to walk, NHS England and Biogen have been able to find a way forward that all parties are happy with and that will ensure Spinraza is available to as many people with SMA as possible.
“There will still be people with SMA who will not be able to access treatment with Spinraza under the terms of this agreement. But we are not closing the door to these people. Uniquely for this type of arrangement we have made a commitment that, during the 5-year course of the MAA, should evidence become available on the potential benefits of Spinraza for type III SMA patients that are currently not included in the MAA, we will review that evidence to see whether it would support a change in the MAA inclusion criteria.”
NICE will now update its draft guidance to reflect the new MAA criteria and this will be issued to consultees for appeal. If no appeals are received, final guidance is expected to be published at the end of July.
NHS England will provide information about the availability of nusinersen, under the terms of the MAA, to the youngest and most severely affected (SMA type 1) patients.
References
Darras et al, 2019 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6541434/